Wednesday, December 23, 2015
I hope you all are enjoying your Winter Break!!!!! As a gift from me to you there is no work for this week (12/21-25). I will put your assignments on-line on Monday (12/28/15). If you are in my 1st block continue to work on your Science Fair Project (due Jan. 4, 2016). Once again enjoy this week off and we will get back to work on 12/28/2015.
Monday, December 7, 2015
12/7/2015

FCAT REFERENCE SHEETS AND PRACTICE ACTIVITIES
Earth Space Science Reference Guide
 |
| Solar Properties |
 |
| Seasons |
 |
| Solar Eclipse |
 |
| Solar Eclipse |

 |
| Rotation and Revolution |
 |
| Layers of the Atmosphere |
 |
| HR Diagram |
 |
| Methods of Heat Transfer |
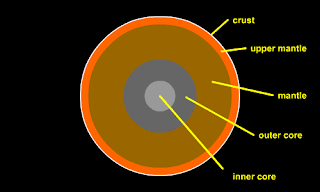 |
| Layers Of Earth (Not to Scale) |
Weather
(Short Term Conditions)
Ex. Today’s high was 930F
On Jan 31, 2000, 50 cm of snow fell
Tomorrow’s humidity will be 30 %
Climate
(Average Conditions over Long Term)
Ex. The average temp for 2008
was 700F
From 1950 – 2000, April was the
month with the highest rainfall
Processes of Scientific Inquiry
-formulation of scientifically
investigatable questions
-construction of
investigations into those questions
-the collection of
appropriate data
-the evaluation of the
meaning of those data
-the communication of this
evaluation
Wednesday, December 2, 2015
12/2/15 and 12/3/15
Topic: Periodic Table
Objectives:
Periodic Table Notes
Topic: Periodic Table
Objectives:
- Describe that as you move from left to right in a period (row) on the Periodic Table, the number of protons increases by one.
- Describe that elements found in the same group/family (column) have the same properties.
- Recognize that elements are grouped in the Period Table of Elements according to their properties.
Periodic Table Vocabulary:
- Atom
- Element
- Proton
- Neutron
- Electron
- Atomic Number
- Periodic Table
- Group or Family (Column)
- Period (Row)
Reference Pages:
- ScienceSaurus: Periodic Table page 265
- Fusion Science Textbook pages 377-387
Periodic Table Literature Connection
Periodic Table Videos
The Periodic Table: Properties of Groups and Periods Periodic Table Video Quiz 1
- Study Jams Periodic Table Video (Students will take the quiz after watching the video).
- Study Jams Elements and Compounds Video (Students will take the quiz after watching the video).
- Brain Pop Periodic Table of Elements (Students will complete the quiz after viewing the video. (Password/Username palmbeach/palmbeach)
Periodic Table Tutorial(s)
Students will copy and then summarize the Periodic Table Notes.
- Atomic Number is defined as the number of protons in the nucleus of an atom.Every atom of the same element has the same number of protons.
- The number of protons determines the identity of the element.
- Typically atoms have the same number of protons, neutrons and electrons.
- The mass number is the total number of protons and neutrons in an atom.
- The periodic table is arranged in horizontal rows of increasing atomic number from left to right. It is also organized into vertical columns with similar characteristics.
- Atoms are composed of protons, neutrons and electrons. These particles can be distinguished based on mass, location and charge.
- Protons: 1 amu (atomic mass unit), in nucleus, positively charged
- Neutrons: approximately 1 amu, in nucleus, neutral charge
- Electrons: negligible mass, outside nucleus, negatively charged.
- Period Number = Number of Energy Levels
- Valence electrons increase moving left to right across the period.
- Groups = Families. Groups have similar properties!
- Acidity increases from Group 1 to Group 18
- Solubility of Metal salts decreases from Group 1 to Group 16
- Metal Salts in water conduct electricity
- Non-metal molecules do not conduct electricity in water
- Solubility is unrelated to element type
Subscribe to:
Comments (Atom)
Date: December 12/17/2019 Topic: Weathering, Erosion, and the Rock Cycle Objective: Identify examples of of suface processes that af...
-
11/20-24/15 Topic: Mixtures and Solutions Objective: Distinguish between Mixtures and Solutions. Give and describe examples of both concep...
-
11/09-10/15 Topic: Intro to matter Mass vs. Weight Video 1 Mass vs. Weight video 2 Mass vs. Weight 3 Objective: Describe the differe...
-
Date : 10/12/2017 Topic: Density Objective: Students will use the density formula to solve problems. Home Learning: Students will comp...




