7th Grade
Date: November 5, 2019
Topic: Temperature
Learning Goal(s):Understand that all objects have temperature due to kinetic energy of their molecules.
Home Learning: Students will complete assignments not finished during class and study for their test on Friday.
Temperature Vocabulary Definitions
Temperature- the average kinetic energy of the particles that make up a material
Average Kinetic Energy- the average kinetic energy in in a material
- Molecule- a group of atoms bonded together, representing the smallest fundamental unit of a chemical compound
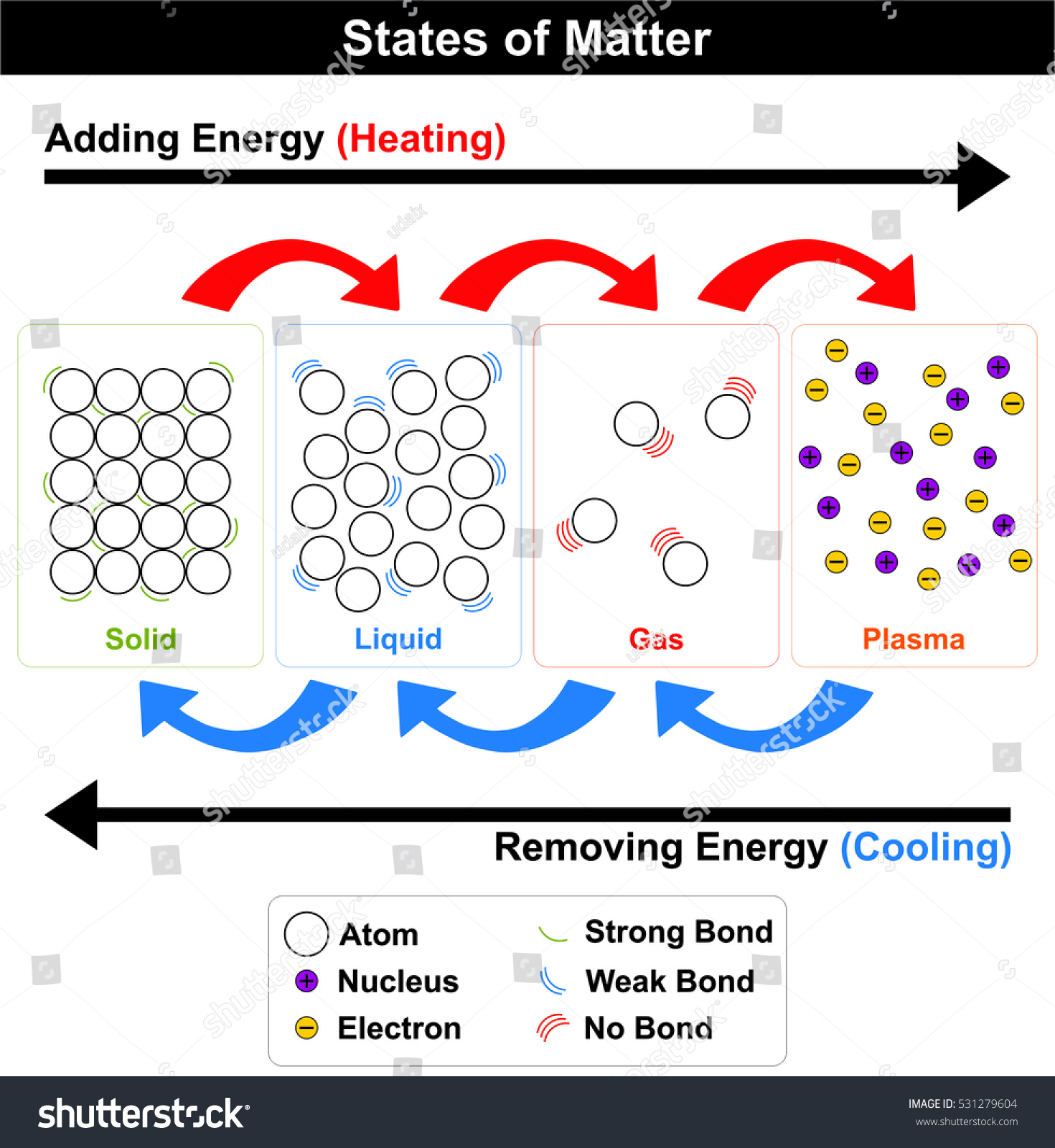
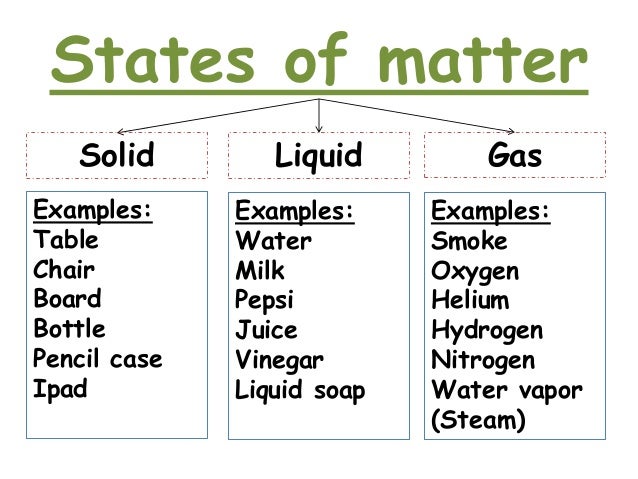
State of Matter- is one of the four distinct forms that matter take on
Liquid- is a substance with a definite volume but not a definite shape.
Gas- is a substance that does not have a definite volume or shape.
Solid- is a substance with a definite volume and shape. Particles are close together and do not move freely.
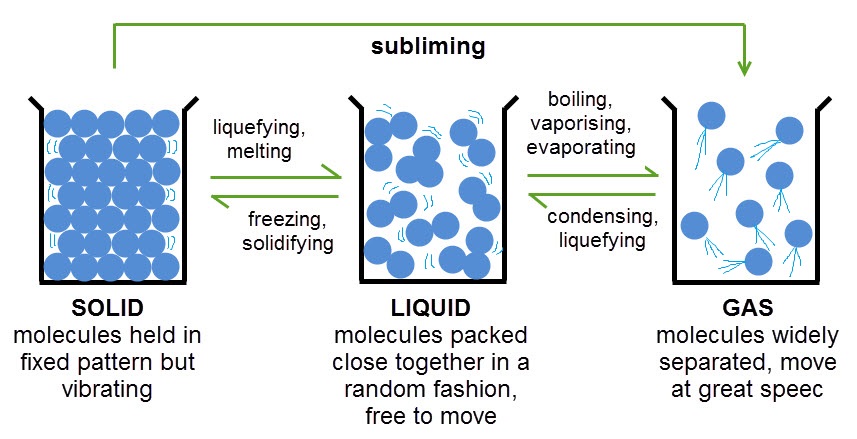
Melting Point-The process in which a solid becomes a liquid
Boiling Point- the temperature at which a liquid boils and turns into vapor
Freezing Point-When temperatures of a liquid are lowered, causing a solid to form.
Law of Conservation of Energy- Energy cannot be created nor destroyed only transformed
Stations
1. Students will watch the following video:
2. Notes:
- Complete Temperature notes on Focus
3. Temperature Flowchart:
- Complete Temperature flowchart on focus
4. Heat and States of matter tutorial:
States of Matter Diagrams