7th Grade
Date: September 25, 2018
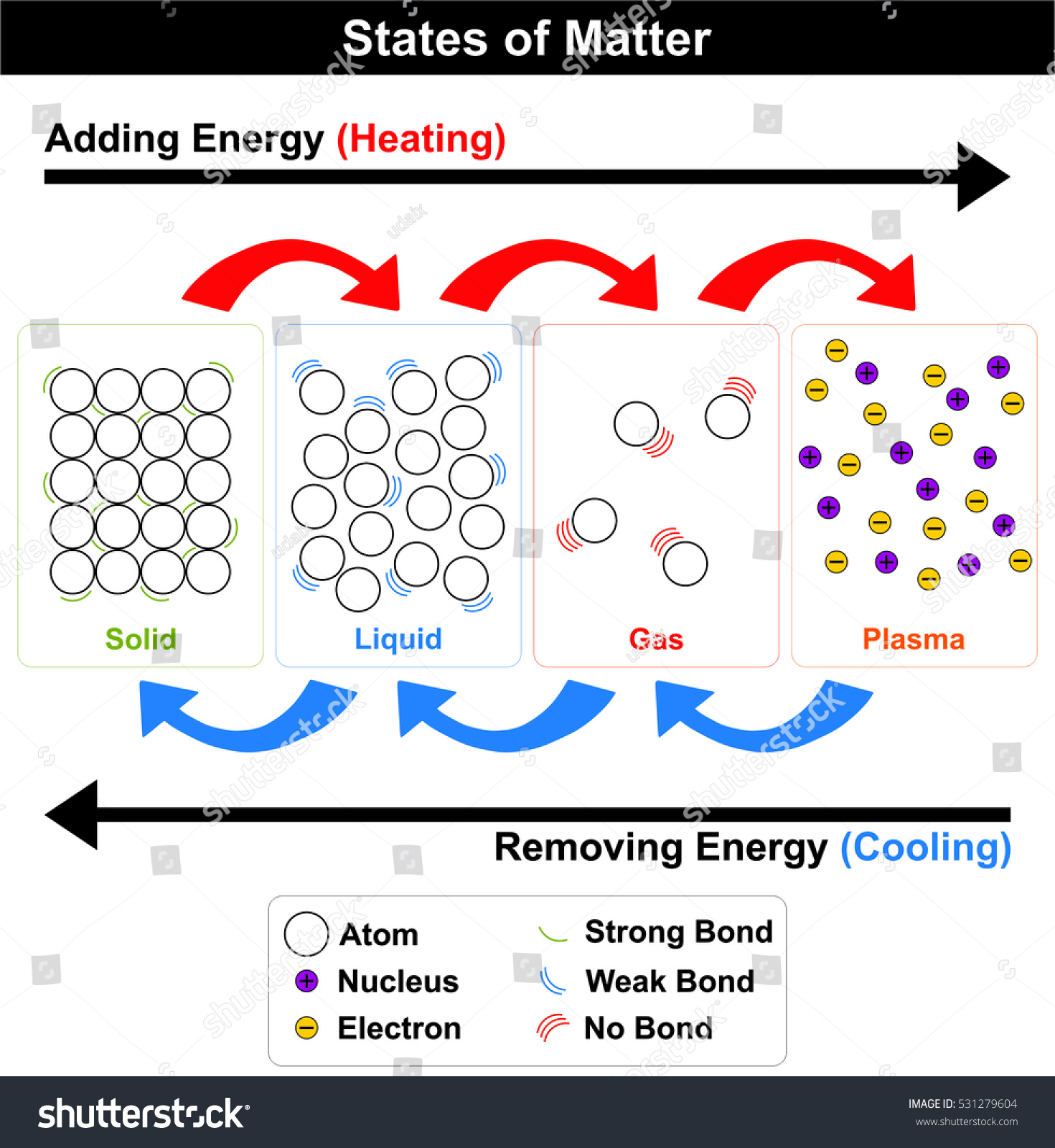
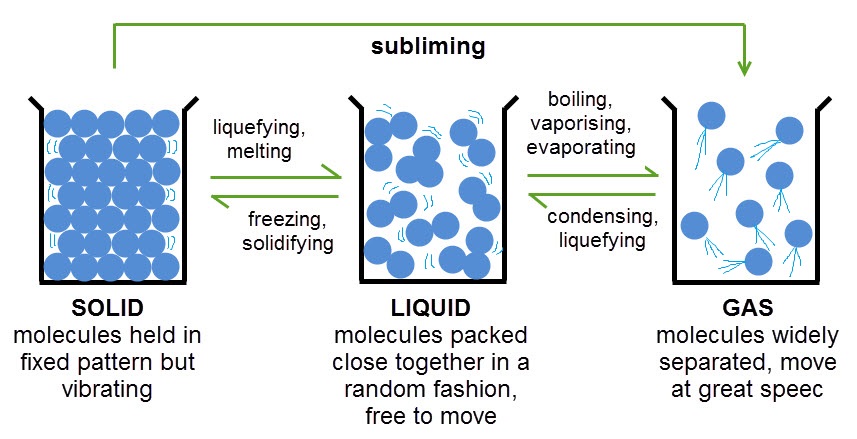
Topic: Temperature
Learning Goal(s):Understand that all objects have temperature due to kinetic energy of their molecules.
Home Learning: Students will complete assignments not finished during class and study for their test on Friday.
Assignment:
- Analyze and answer the following scenario in their Interactive Journal: A cup of coffee is so hot that it cannot be drunk. As a result, an ice cube is put into the coffee. What direction is the energy moving? When would the energy flow stop?
8th Grade
Date: September 25, 2018
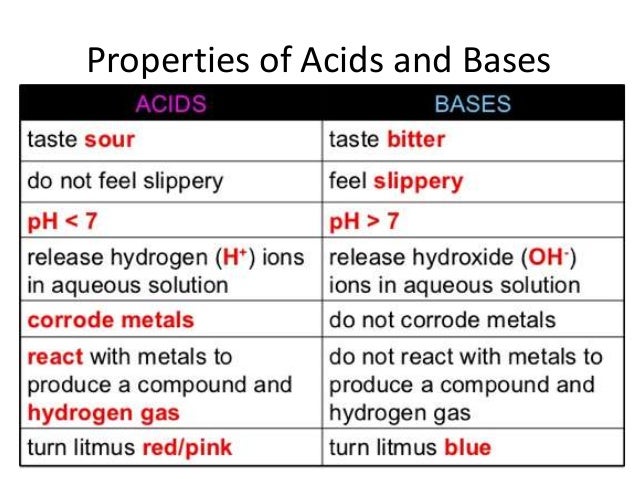
Topic: Acids, Bases, and Salts
Learning Goals:
Topic: Acids, Bases, and Salts
Learning Goals:
- Differentiate between acids and bases.
- Identify examples of acids and bases.
- Distinguish where acids and bases fall on the Ph scale (Potential Hydrogen Scale).
Home Learning: Students will complete vocabulary index cards and study their Cornell notes
Assignment:
- Students will complete an on-line acids, bases, and salts lab. Students will write the answers from the properties table in their Interactive Journal.
- Click the following to began the on-line lab: http://education.abc.net.au/home#!/media/1390445/ph-acids-and-bases